Pharmacovigilance Services & Solutions

Comprehensive Pharmacovigilance Services Tailored to Your Needs

End-to-End Pharmacovigilance Support

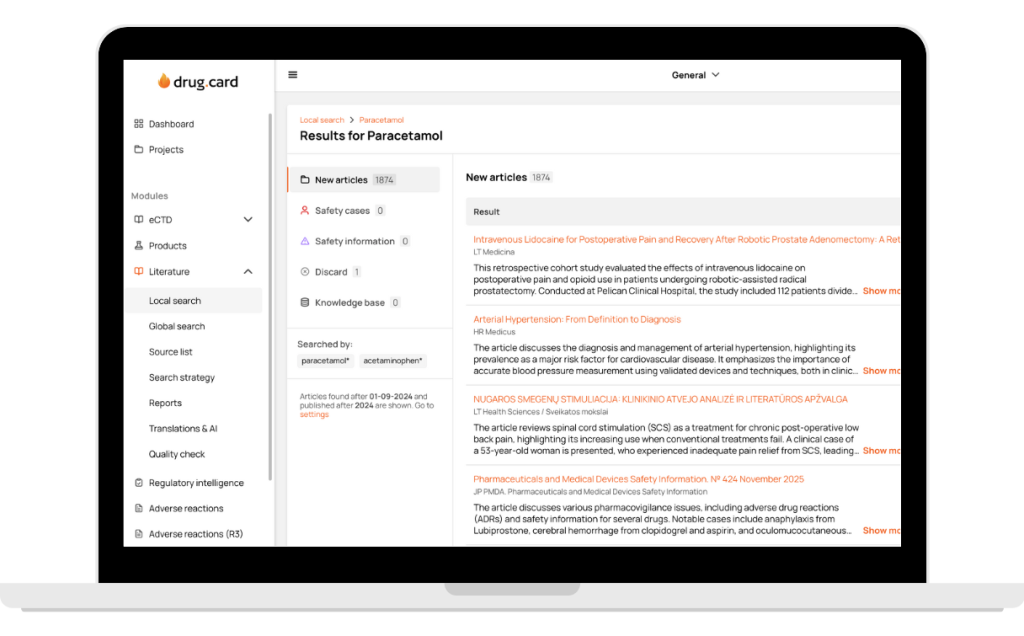
Retrospective Searches
Retrospective medical review helps recover historical drug safety and pharmacovigilance data by reviewing past literature, regulatory updates, and reference sources. Such drug safety services ensure patient safety so no relevant information is missed and your pharmacovigilance documentation is complete and accurate which supports audits and inspections, and strengthens the completeness and accuracy of your pharmacovigilance documentation.
Focused Scalable Solutions for Pharma
Ensure Compliance with Us
our team
Full Set of Pharmacovigilance Solutions for Different Business Types
Our Core Pharmacovigilance Service Offerings
Our pharmacovigilance professionals provide reliable global literature monitoring through PubMed. We also cover local literature across 108 countries, ensuring compliant, consistent literature surveillance aligned with regional pharmacovigilance requirements.
Case Processing & Individual Case Safety Reports (ICSRs)
Local Safety Officer (Local QPPVs)
Signal Detection
Regulatory Intelligence
Reference Products Monitoring
Medical Writing
We provide medical writing services to create clear, compliant safety documentation including narratives, reports, and regulatory materials.
Periodic Safety Documentation Management (PSUR, DSUR, Risk Management plans)
Pharmacovigilance Training & Consultancy
Why Choose DrugCard as a Pharmacovigilance Service Provider
- Proprietary PV Technology – our in-house platform streamlines monitoring, reduces manual workload, and increases operational efficiency.
- AI-Enhanced Article Evaluation – automated assistance speeds up literature assessment while improving consistency and accuracy.
- Integrated Data Workflows – literature insights link directly to our safety database, enabling automatic pre-filling of key fields.



DrugCard Is a Team of Experienced Pharmacovigilance and IT Experts
DrugCard’s founders bring over 10 years of experience in drug safety and pharmacovigilance, regulatory affairs, and software development. We ensure global coverage & regulatory compliance for patient safety.
How Our Pharmacovigilance Services Work
1. Simple Onboarding Process
2. Dedicated Account & Project Management
3. Transparent Reporting & KPIs
Benefits of Outsourcing Pharmacovigilance Services
Cost-efficient – our proprietary monitoring platform automates routine PV tasks and reduces manual workload, allowing us to deliver high-quality services at a lower operational cost.
Transparent – clients receive direct access to reports, activity logs, and compliance KPIs, providing full visibility into ongoing pharmacovigilance activities aligned with their SOPs.
Flexible – both our platform configuration and team structure can be adapted to specific products, markets, reporting frequencies, and internal workflows.
Fast start – projects are onboarded within two business days, with the first monitoring results delivered within one week.