AI-Powered Adverse Event Database
Revolutionizing Adverse Event Management with AI
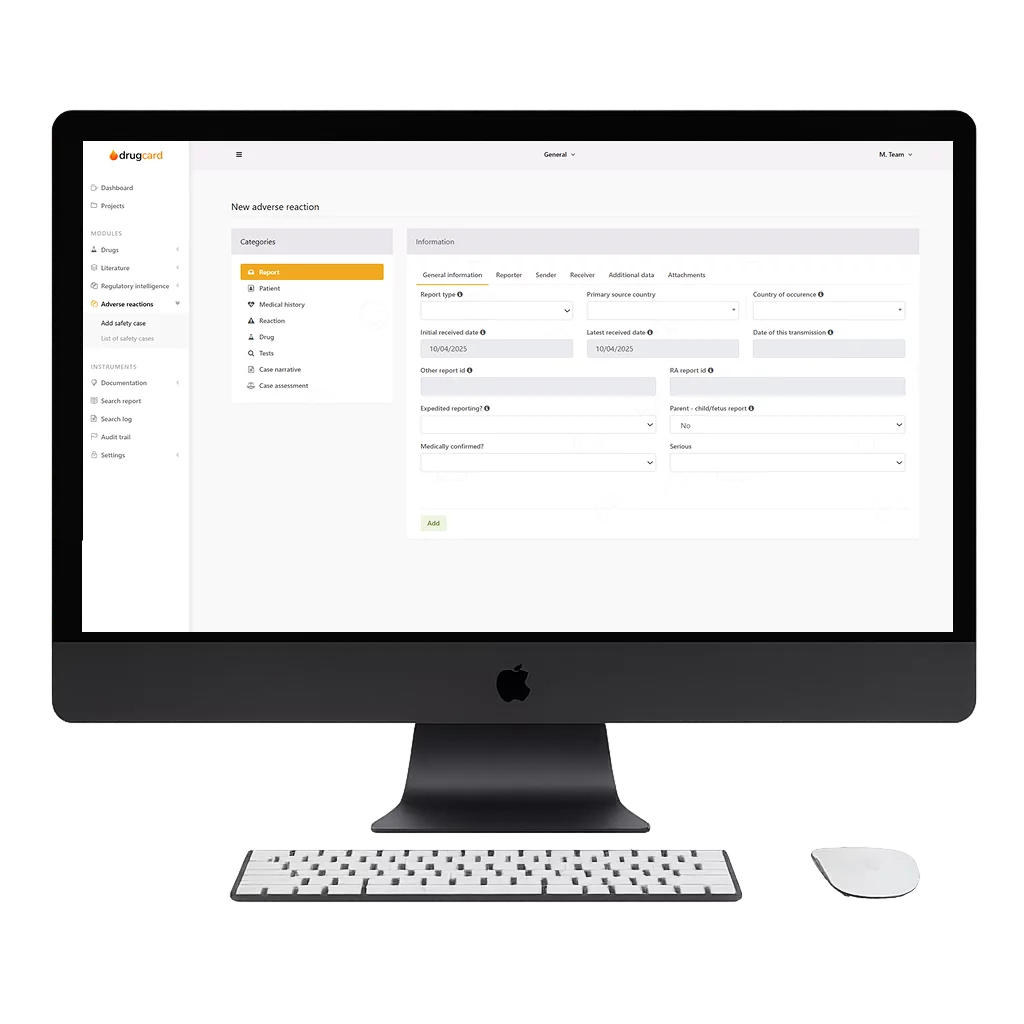
Our platform brings the next generation of pharmacovigilance, automating adverse event collection, structuring data, and ensuring seamless regulatory compliance with ICH E2B(R2) and E2B(R3) standards.

Centralized, structured, and instantly accessible – ensuring seamless regulatory submissions with zero delays.

Effortlessly generate and submit reports in both E2B(R2) and E2B(R3) formats, meeting the latest compliance requirements worldwide.
AI-powered signal detection and risk assessment reduce manual effort, highlight safety trends, and ensure proactive decision-making.

Compliance with FDA CFR 21 Part 11
for electronic records and signatures. A certificate of conformity allows you to use the system officially in the EU, the US, and CIS markets.

Data centers with a certified information security management system according to ISO / IEC 27001:2013 and DigitalOcean SOC 2 Type II.

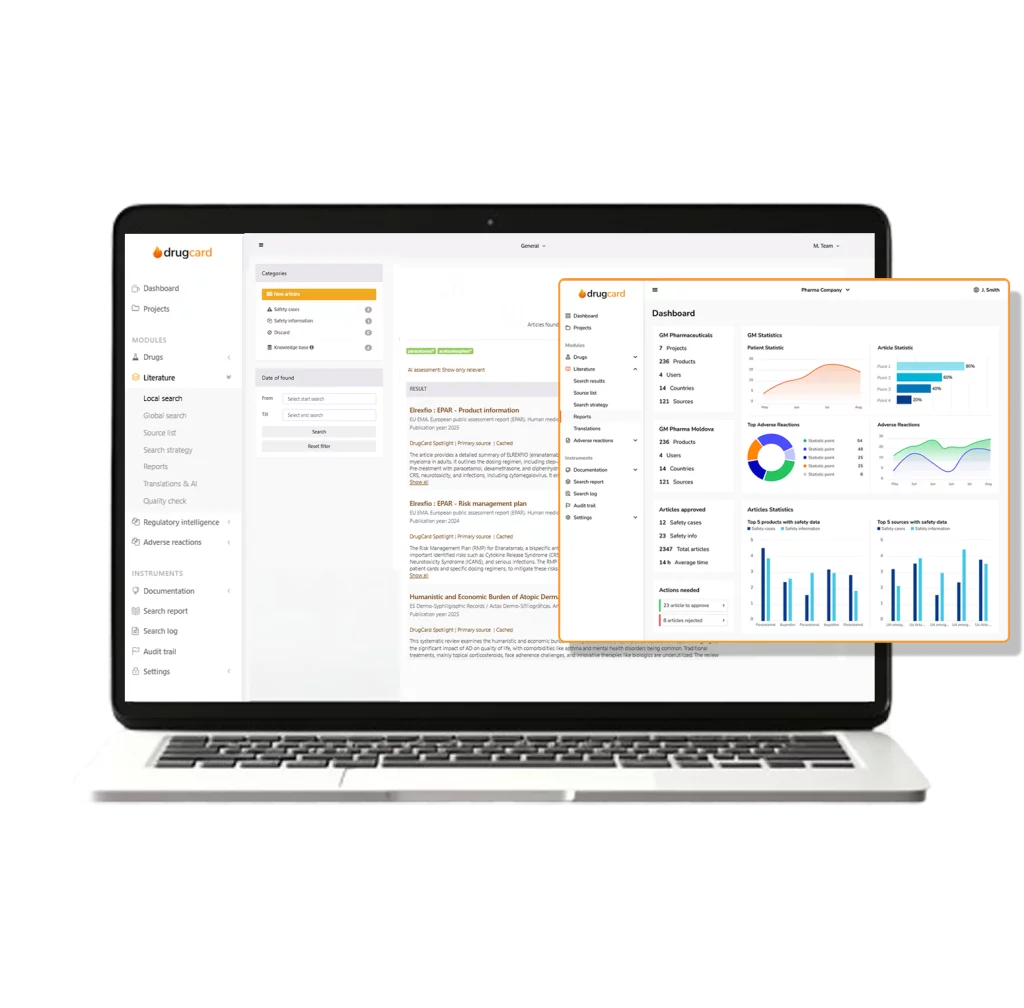
Log in anytime, anywhere. AI keeps your literature monitoring fully automated – no manual searches, no downloads, just instant insights.
Switch Instantly
AI captures, structures, and processes adverse event reports from multiple sources in seconds.
Identify duplicate cases, assess severity, and detect emerging safety trends in real-time.
Convert reports into E2B(R2) or E2B(R3) formats instantly and submit them to regulatory authorities without hassle.
- User access roles control
- E2B R3/ R2 XML files
- Audit trail
- Book a meeting for the demonstraion
- Anwers your additional questions
- Implenetation DrugCard to your pharmacovigilance system
- Tariff plans, pricing, addional features
