AI Powered Regulatory Intelligence Software
Stay Ahead with Real-Time Regulatory Intelligence Solution
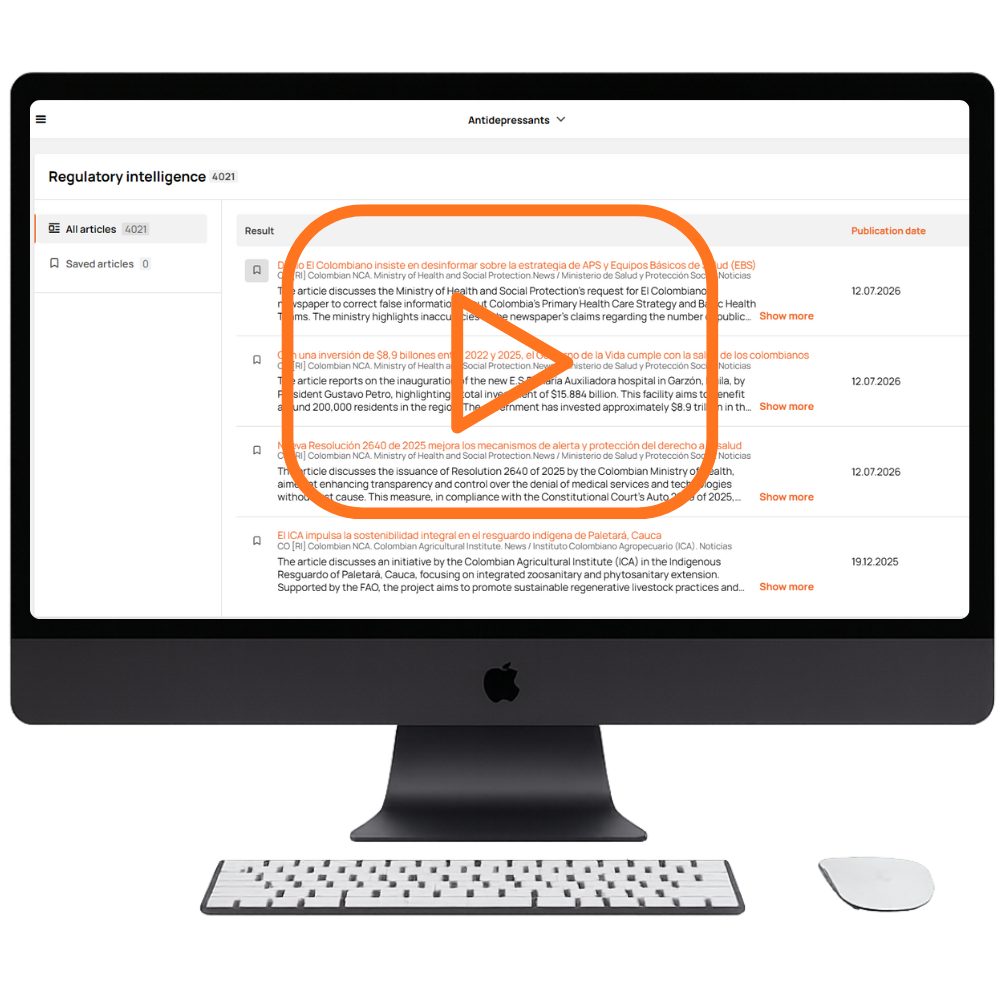
DrugCard’s regulatory intelligence software aggregates regulatory changes from global and local authorities, ensuring your team never misses critical regulatory insights in safety or compliance requirements.
Key Features of Our Regulatory Intelligence Platform

Complete Regulatory Data Visibility
Our platform provides regulatory information across 40 countries with 1-day onboarding for new markets. You receive real-time alerts, continuous tracking of regulatory intelligence updates from regulatory agencies along with the ability to save key publications to Favorites for instant market access.
Advanced Search & Discovery
A unified cross-site search that works across differently structured regulatory websites, combined with targeted keyword filtering for deeper insights. Designed to enhance regulatory intelligence and strengthen regulatory compliance, it offers fully customizable update frequency — far beyond standard weekly cycles.

Seamless Platform Integration
Native integration with other DrugCard modules for an end-to-end monitoring regulatory updates and getting timely insights. API options to connect with your existing compliance systems, ensuring compliance and operational efficiency.

Security, Compliance & Data Integrity

Continuous Access & Automation
Comprehensive Data You Can Rely On
How Our Regulatory Intelligence Database Works
Who Can Benefit from the Database
Why Choose DrugCard AI-Powered Regulatory Intelligence?
- We aggregate updates from all relevant regulators so you no longer have to open dozens of pages.
- A clean feed with filters by authority, topic, and country — no extra clicks.

- Book a meeting for the demonstraion
- Anwers your additional questions
- Implenetation DrugCard to your pharmacovigilance system
- Tariff plans, pricing, addional features