AI-Powered Literature Monitoring
Automate global and local medical literature monitoring, eliminate manual pharmacovigilance literature search so your PV team can focus on real patient safety work, not routine.
Key Benefits of Our AI-Driven Literature Monitoring Platform
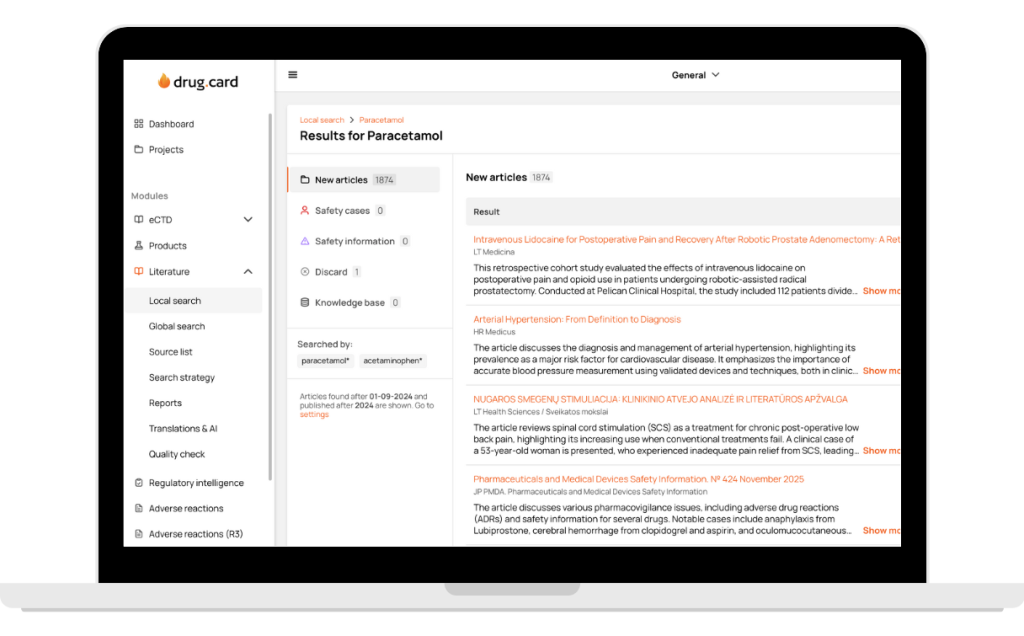
DrugCard unifies global and local publications into one consistent, standardized process. The same workflow, the same quality, and the same reliable automation — whether you track PubMed full texts or local non indexed journal sources.

Complete Global & Local Coverage
All medical sources in one platform — from global literature full-text monitoring to 108 countries and 2174+ local sources. Every client gets a tailored source list, country-specific recommendations, and fast addition of any journal without bureaucracy.

Higher Impact With the Same Team
Automation removes repetitive literature screening so your experts can focus on real PV work — signal detection, risk assessment, drug safety documentation, and scientific evaluation.
Zero Missed Publications, Zero Compliance Risk
The platform captures all published articles and scientific literature automatically — no human errors. All results are fully traceable so nothing is overlooked.

Full Visibility and Built-In Documentation
You see every task, action, and screening step in real time, eliminating blind spots. DrugCard automatically records all activities, providing complete traceability and ready-to-present audit evidence without manual paperwork.

Data Integrity and Security
Your PV data is protected by FDA 21 CFR Part 11–compliant records and signatures, plus a certificate of conformity for use in the EU, US, and CIS. Hosting on ISO/IEC 27001:2013 and SOC 2 Type II infrastructure ensures enterprise-grade security.

Always-On Access, Anywhere
As a SaaS solution, DrugCard ensures uninterrupted, 24/7 access on any device. Your work continues smoothly, even across teams, regions, and time zones.
Does this sound like a solution that could help your team right now?
We invite you to experience the benefits of DrugCard with a free 2-week trial.
DrugCard Platform in Numbers
121+
Countries
Medical journals coverage
Medical journals coverage
2200+
Local journals
Continuously monitored
Continuously monitored
~2
Weeks
New country Easily Added
New country Easily Added
Comprehensive Feature Set for Literature Screening
AI Article Summaries
Artificial intelligence instantly generates summaries so you can understand the relevant articles’ content without reading the full text.
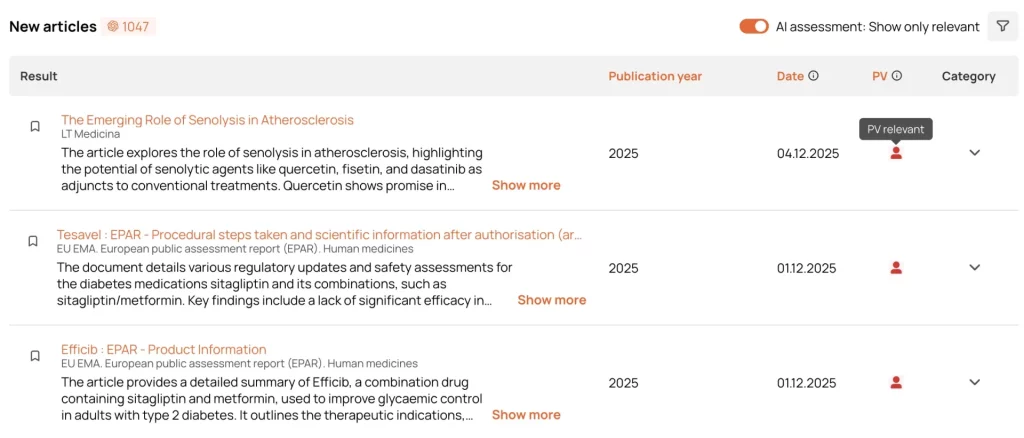
AI Pre-Assessment of Safety Relevance
Artificial intelligence identifies whether an article contains any safety-relevant references, safety signals, and prioritizes it.
One-Click Case Creation From Literature
Convert an article into an individual case safety report in a single click. Fully integrated with the DrugCard adverse event database.
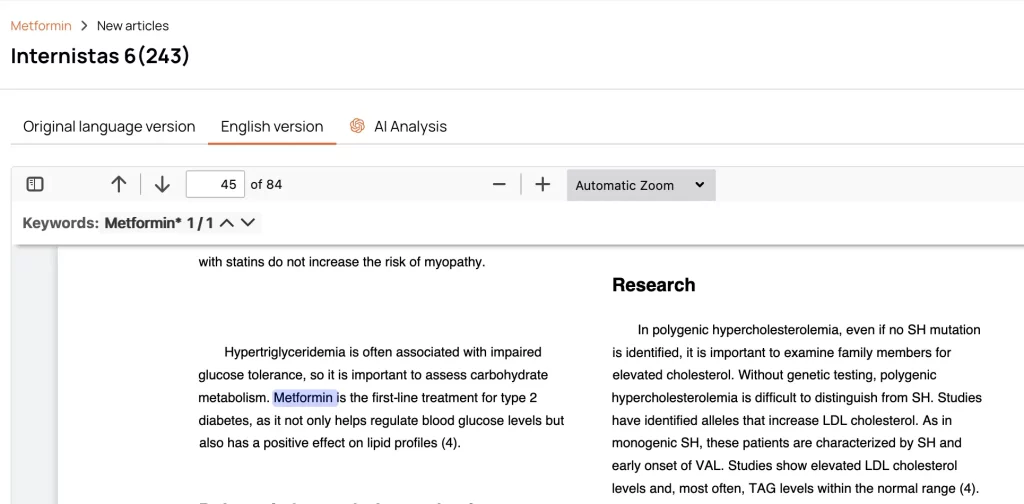
Automatic Translation of Local Sources
Over 100 supported languages ensure that local literature is just as accessible as global literature — no external translation needed.
Validated, Audit-Ready Platform
Our literature monitoring services successfully passed inspections and audits. Reliability proven by regulatory authorities.
Flexible Project Configuration
Configure literature search projects for any setup — multi-country, multi-client, diverse roles, source lists, and requirements.

A Shift From Manual Reading to Automated Medical Literature
The problem with manual screening
Manual literature review consumes hours of repetitive literature surveillance and regulatory reporting, increases the risk of human error and burnout, and makes traceability difficult.
How DrugCard solves it
As the only SaaS tailored to your local and global literature search needs, we offer automated AI relevance checks, summaries, keyword highlighting, weekly reports, and real-time notifications plus continuously expanding coverage.
How It Works
1. Create your project
Select countries, define your coverage (whether it’s global searches, or local publications), and identify the products you want to monitor.
2. Get instant results
The system processes sources immediately — no manual runs or waiting time for search results.
3. Stay updated automatically
Receive real-time notifications about new findings and weekly automated reports for full oversight.
Why Leading Pharmacovigilance Teams Choose Us
Teams rely on us for dependable literature surveillance that saves time and supports safety work across all therapeutic areas.
Unmatched Expertise in Literature Screening
Experts in literature review, drug safety, and medicinal products' safety signals — delivering the accuracy your PV processes rely on.
Full Compliance with GVP & Data Security
The system includes all essential controls for GxP activity and good pharmacovigilance practice. You get comprehensive, structured results; role-based user access control; automatic aggregate reports, including search strategy documentation; and a complete audit trail. There’s no need to download or review files manually — the system handles it for you.