Adverse Event Software for Efficient Safety Data Management
Key Features of Our Adverse Event Database

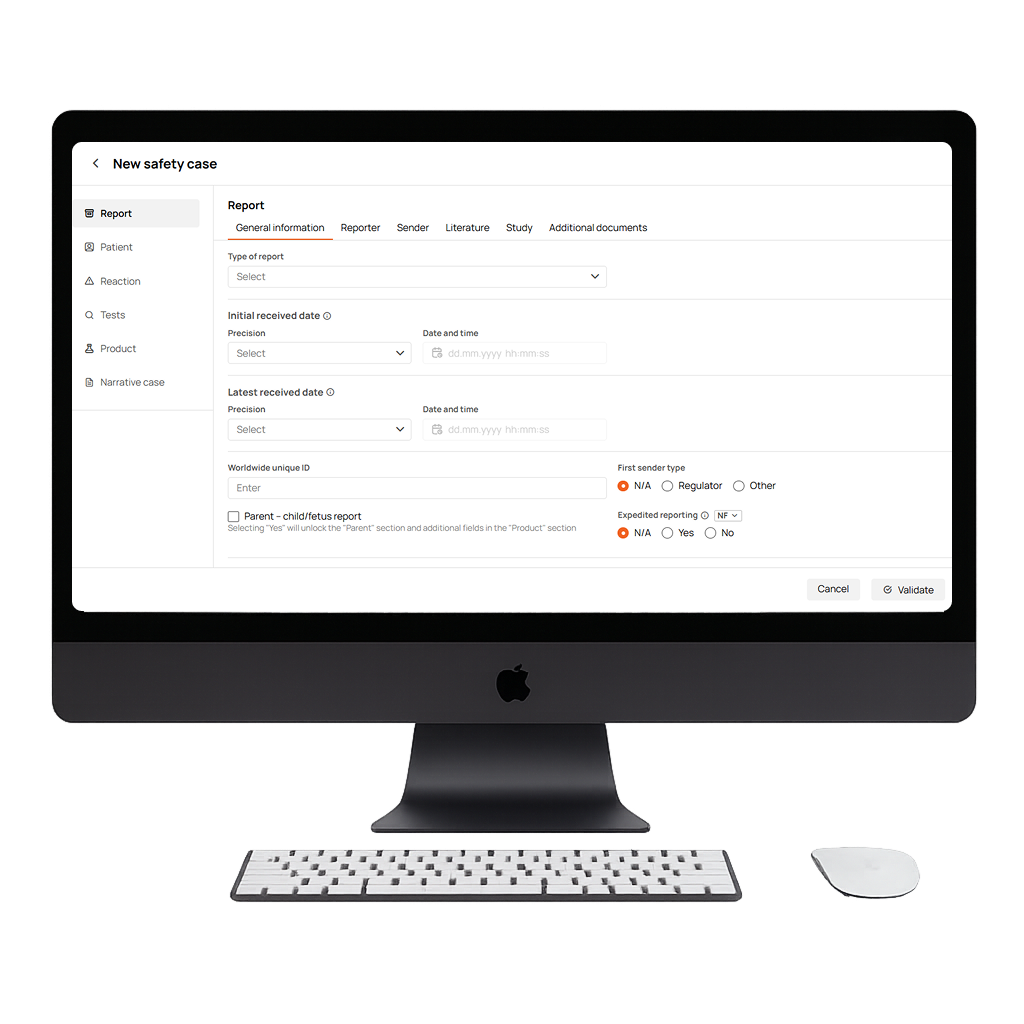
Lightning-Fast Centralized Adverse Event Data Collection
An intuitively structured, submission-ready drug safety database with all required fields in one place. Centralized, instantly accessible, and optionally connected to DrugCard’s literature screening module to make data entry easier and reduce manual work.

Multi-Format Support (E2B R2 & R3)
A flexible adverse event management system that supports both E2B(R2) and E2B(R3) formats, with full back-and-forth conversion ensuring globally compliant reporting and compatibility across evolving pharmacovigilance regulatory standards.
AI-Powered Signal Detection
An AI-enhanced adverse event management solution that reduces manual data entry and eases case intake, highlights safety trends, and simplifies adverse event detection while capturing, structuring, and processing reports from multiple sources and helping complete missing information from literature to help you implement corrective actions faster.

Data Integrity

Secure and Compliant Data Management

An adverse event management tool that offers CIOMS I, CIOMS II, line listings, and ADR summary tabulations enabling fast export of critical safety information across multiple formats for deeper analysis and regulatory-ready documentation.
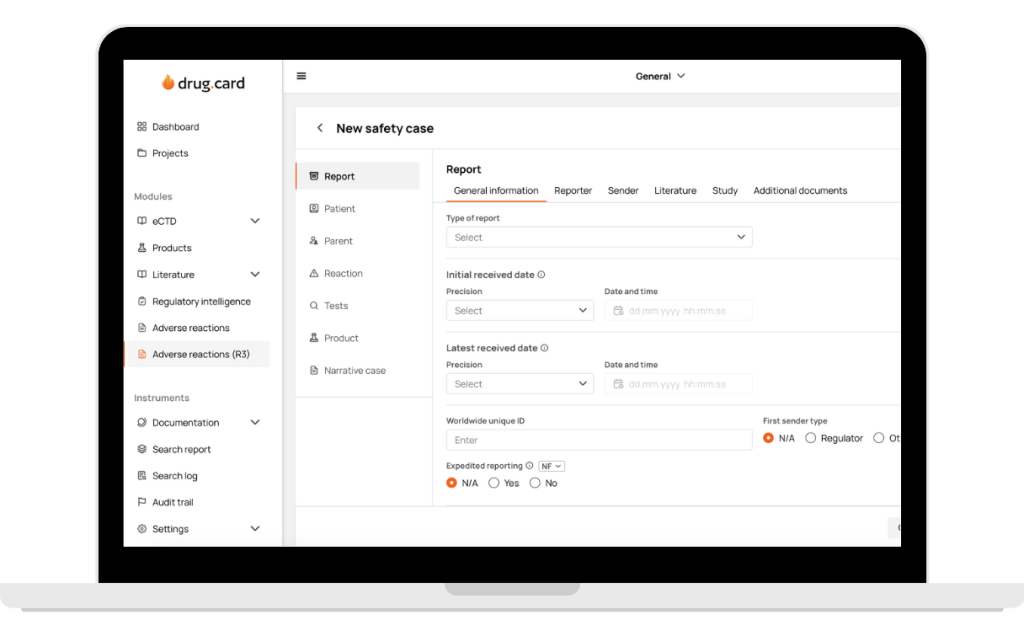
How Does It Work?
Switch Instantly
Benefits of Using Our Adverse Event Detection Platform
Intuitive & Fast to Use
An adverse event software with user-friendly interface that requires no lengthy training and integrates quickly into PV workflows, is highly convenient for healthcare professionals to use.
Less Manual Work
Automatic pre-filling from literature reduces manual data entry and speeds up case processing, making it easier to identify trends.
Flexible Data & Reporting
Export data in multiple formats for seamless integration into reports and analysis.
Automated Aggregate Outputs
Automatic generation of aggregate reports without manual preparation or extra effort for efficient risk management.
Full Compliance and Regulatory Alignment with GVP
- User access roles control — role-based permissions and restricted access to ensure secure, compliant workflows.
- E2B(R2/R3) XML file handling — import, export, and validation of safety cases in both formats for your regulatory reports.
- Full audit trail — time-stamped tracking of every action, change, and user for complete traceability.
- Book a meeting for the demonstraion
- Anwers your additional questions
- Implenetation DrugCard to your pharmacovigilance system
- Tariff plans, pricing, addional features
